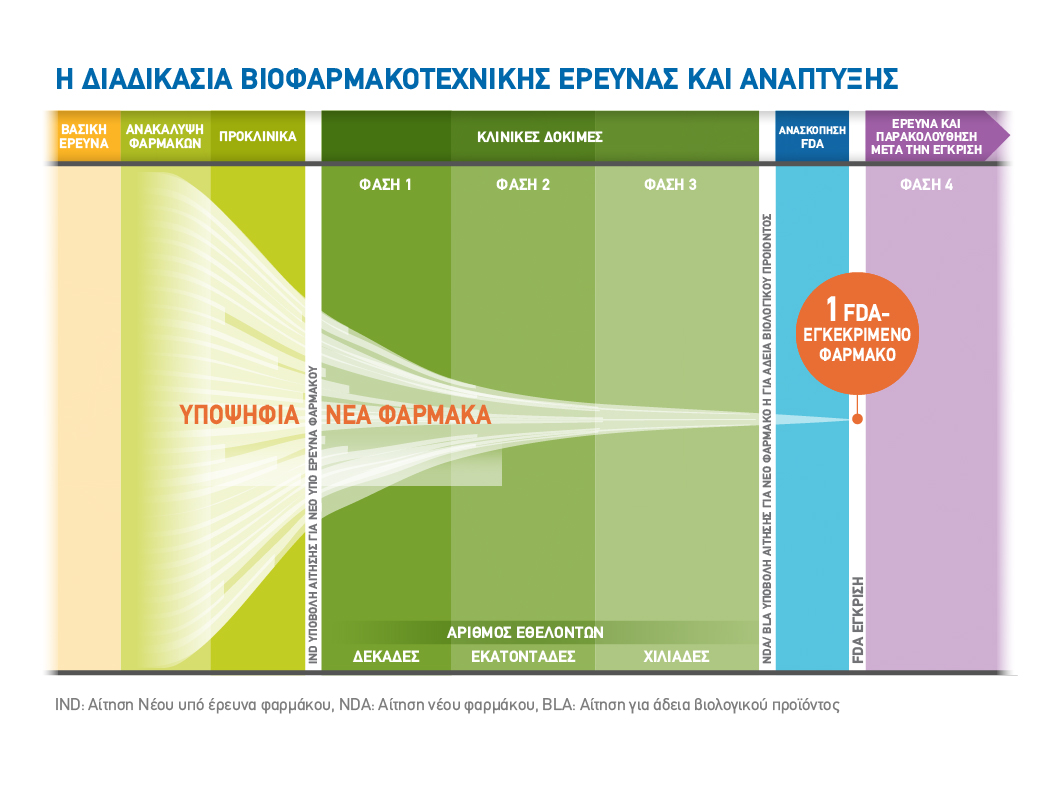
Η ανακάλυψη μίας νέας θεραπείας ξεκινά με την κατανόηση της ασθένειας σε βάθος. Η βασική επιστημονική έρευνα προσφέρει ενδείξεις για το πως και με ποιους τρόπους μπορούμε να στοχεύσουμε τα συμπτώματα και τα αίτια μίας ασθένειας. Έχοντας μία βασική ιδέα υπόψη, οι επιστήμονες εργάζονται για να κατανοήσουν τους πιθανούς στόχους για τη δημιουργία ενός φαρμάκου. Τέτοιοι στόχοι μπορεί να είναι μία πρωτεΐνη, το RNA, το DNA ή άλλα στοιχεία τα οποία εμπλέκονται στην εμφάνιση ή εξέλιξη μίας νόσου. Οι ερευνητές διενεργούν μελέτες σε κύτταρα, ιστούς και ζώα για να καθορίσουν εάν ο βιολογικός στόχος επηρεάζεται από το φάρμακο. Στη συνέχεια αναζητούν μία νέα δραστική ουσία– ένα υποσχόμενο μόριο το οποίο θα μπορούσε να επηρεάσει το βιολογικό στόχο και, πιθανά, να εξελιχθεί σε μία νέα θεραπεία. Αυτό είναι το πρώτο βήμα στο «ταξίδι» της ανακάλυψης και ανάπτυξης το οποίο ξεκινά με την αρχική έρευνα και καταλήγει στη διάθεση θεραπειών που σώζουν και βελτιώνουν τις ζωές μας1.
Η διαδικασία Έρευνας και Ανάπτυξης
βιο-φαρμακευτικών σκευασμάτων
Προκειμένου να αντιμετωπιστούν περαιτέρω ζητήματα αβεβαιότητας σχετικά με τα οφέλη και τους κινδύνους μιας νέας θεραπείας, αυτή αξιολογείται στο πλαίσιο μιας κλινικής δοκιμής. Πριν ξεκινήσει η διαδικασία κλινικής μελέτης συνήθως σε τρία στάδια, μια εταιρεία ή ακαδημαϊκό ίδρυμα πρέπει να υποβάλει ένα ερευνητικό πρωτόκολλο προς αξιολόγηση και έγκριση στις κανονιστικές αρχές.
Η κλινική έρευνα ξεκινά με μια μελέτη σε λίγους υγιείς εθελοντές (φάση 1) πριν προχωρήσει σε ολοένα και μεγαλύτερες μελέτες φάσης 2 και φάσης 3, οι οποίες συχνά περιλαμβάνουν μεγάλο αριθμό ασθενών και διεξάγονται σε πολλά κέντρα και διάφορες χώρες και περιοχές. Στόχος αυτών των μελετών είναι η διερεύνηση της ασφάλειας και της αποτελεσματικότητας του νέου φαρμάκου. Όλες οι δοκιμές καταχωρούνται σε δημόσια διαθέσιμες βάσεις δεδομένων πριν από την έναρξη τους2.
Οι κλινικές δοκιμές αποτελούν το μεγαλύτερο μέρος της διαδικασίας έρευνας και ανάπτυξης για ένα νέο φάρμακο, και απαιτούν πολύ χρόνο και σημαντικούς πόρους για την πραγματοποίηση τους.
Οι βιο-φαρμακευτικές εταιρίες υποστηρίζουν και διεξάγουν το μεγαλύτερο μέρος αυτής της σημαντικής εργασίας. Χωρίς αυτές, δεν θα μπορούσαν να εγκριθούν νέα φάρμακα και –το σημαντικότερο– να διατεθούν σε ασθενείς που τα χρειάζονται.
Από την ανακάλυψη ενός νέου φαρμάκου μέχρι την έγκρισή του από τους ρυθμιστικούς φορείς το ταξίδι είναι μακρύ. Η ανάπτυξη ενός νέου φαρμάκου διαρκεί κατά μέσο όρο τουλάχιστον 10 χρόνια ενώ κοστίζει κατά μέσο όρο 2,6 δισεκατομμύρια δολάρια. Αξίζει να σημειωθεί ότι λιγότερο από το 12% των υποψήφιων φαρμάκων που ερευνώνται σε κλινικές δοκιμές Φάσης 1 θα εγκριθούν από το FDA και άλλες κανονιστικές αρχές.
Από το 2000, οι εταιρείες μέλη της PhRMA έχουν επενδύσει πάνω από 800 δισεκατομμύρια δολάρια στην έρευνα και ανάπτυξη νέων θεραπειών μόνο στις ΗΠΑ3.